Comparative effectiveness and personalized medicine research using real-world data
Background

IMI GetReal: Incorporating real-life clinical data into drug development
- GetReal aimed to show how robust new methods of real-world evidence collection and synthesis could be adopted earlier in pharmaceutical research and development and the healthcare decision-making process.
- Together with Bern, we investigated statistical methods for network meta-analysis
ISCB Utrecht

Introduction
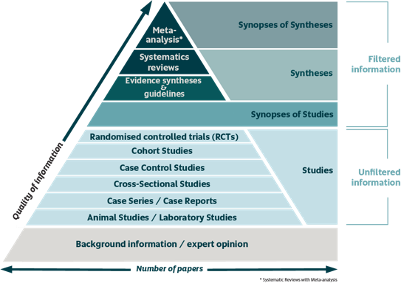
Randomization evenly distributes measured and unmeasured factors among intervention groups and thereby
- Facilitates identification of causal relationships
- Avoids bias and confounding
- Offers a framework for assessing efficacy
The results of randomized, controlled trials are considered to be evidence of the highest grade
The Hierarchy of Evidence Pyramid
Limitations of randomized trials



Limitations of randomized trials
- Experimentation may be unnecessary
- Experimentation may be inappropriate
- Experimentation may be impossible
- Experimentation may be inadequate
The efficacy-effectiveness gap
Variability in drug response may arise due to
- Heterogeneity in case-mix (e.g., comorbidities)
- Heterogeneity in concomitant medications
- Heterogeneity in treatment adherence
- Heterogeneity in prescription behaviour
The efficacy-effectiveness gap
Information from RCTs can only answer a minuscule fraction of the near-infinite number of questions about subpopulations, interactions, treatment settings, effects, etc., that are relevant to patients and healthcare professionals at the point of care
Real-world data and Real-world evidence
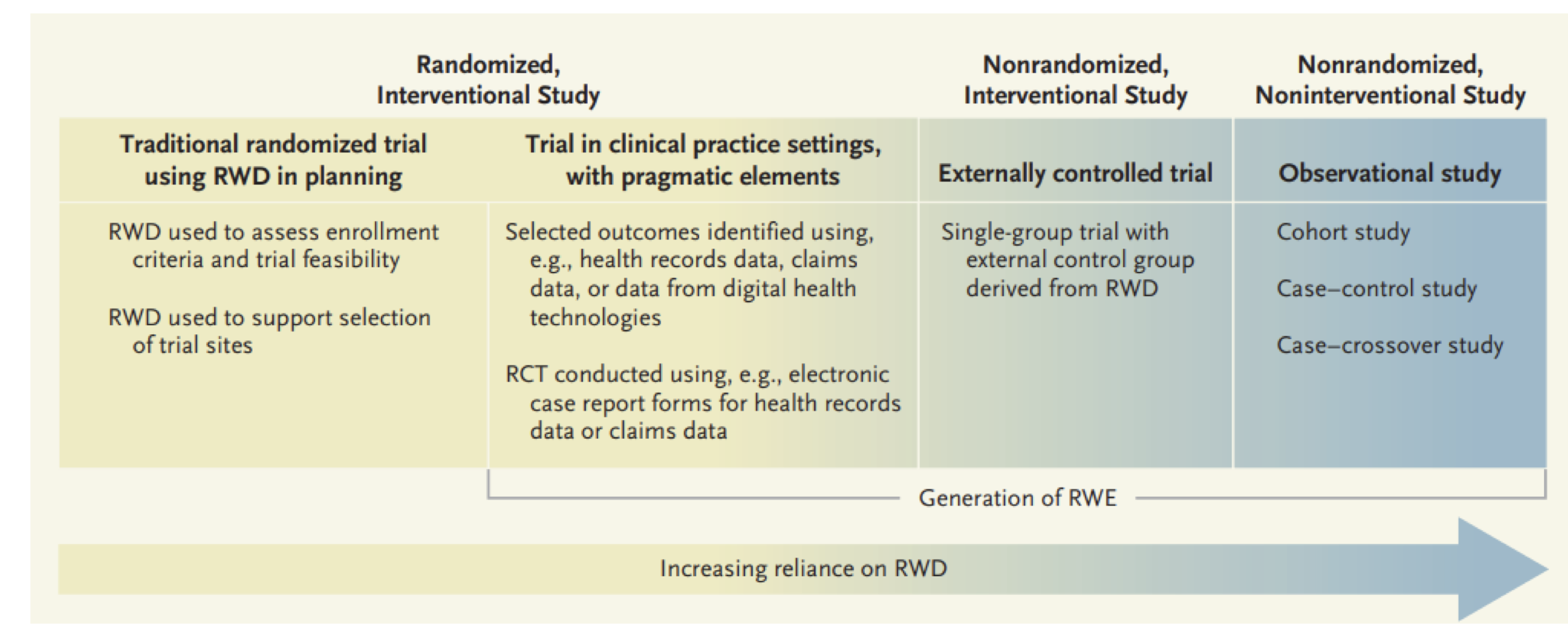
Randomized trials and real-world data
- The Achilles heel of experimental studies is sample size and the lack of random selection
- The Achilles heel of observational studies is error due to imbalance in (un)observed variables
‘’Real-world data can improve our understanding of health and social care delivery, patient health and experiences, and the effects of interventions on patient and system outcomes in routine settings’’ (NICE)
What is real-world data (RWD)?

Data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources
Routinely collected data relating to a patient’s health status or the delivery of health care from a variety of sources other than traditional clinical trials

Data collected outside the context of a highly controlled clinical trial
What is real-world data (RWD)?
RWD is commonly understood as observational data
- Primary use of data (e.g., data collected in an observational study, data collected with digital wearable devices or patients’ or physicians’ surveys)
- Secondary use of data (e.g., electronic healthcare records, claims data, registries)
- A combination of both
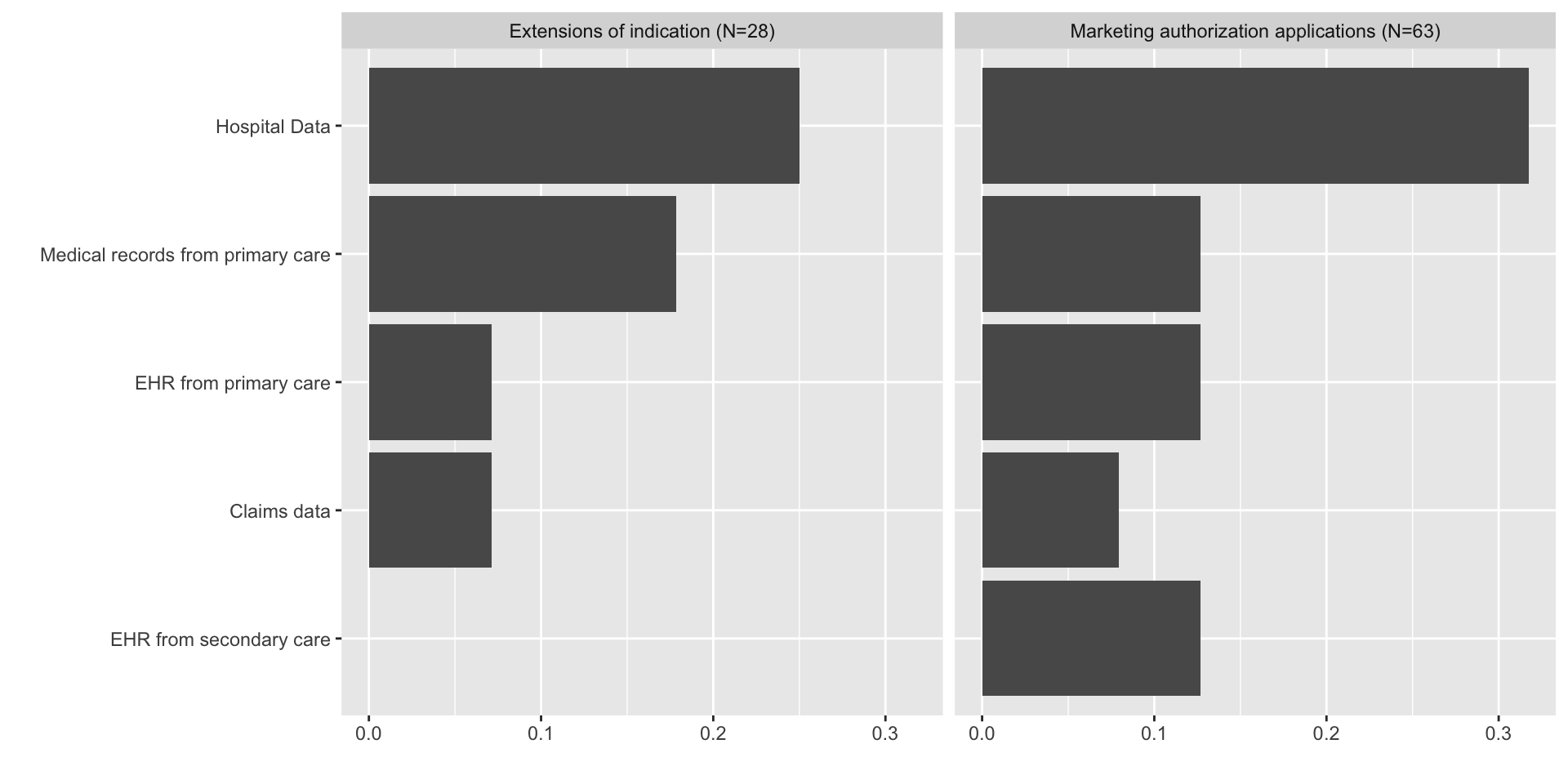
Sources of real-world data
Studies generating real-world data
Real-world data is not new in medical decision making
- Phase IV trials
- Post-authorisation safety studies (PASS)
- Post-authorisation efficacy studies (PAES)
- Studies to measure the effectiveness of risk minimisation measures (RMMs)
- Studies to support life cycle benefit-risk evaluation
How is real-world data used?
- Explore and quantify the unmet medical need within a particular disease area
- Derive information on the current standard of care in different jurisdictions
- Augment or replace the concurrent control of a randomized clinical trial
- Monitor long-term safety and effectiveness
- Tailor the usage, schedule and dosage of a treatment to an individual patient’s needs
What is real-world evidence (RWE)?
Evidence generated from the analysis of real-world data
How is RWE used?
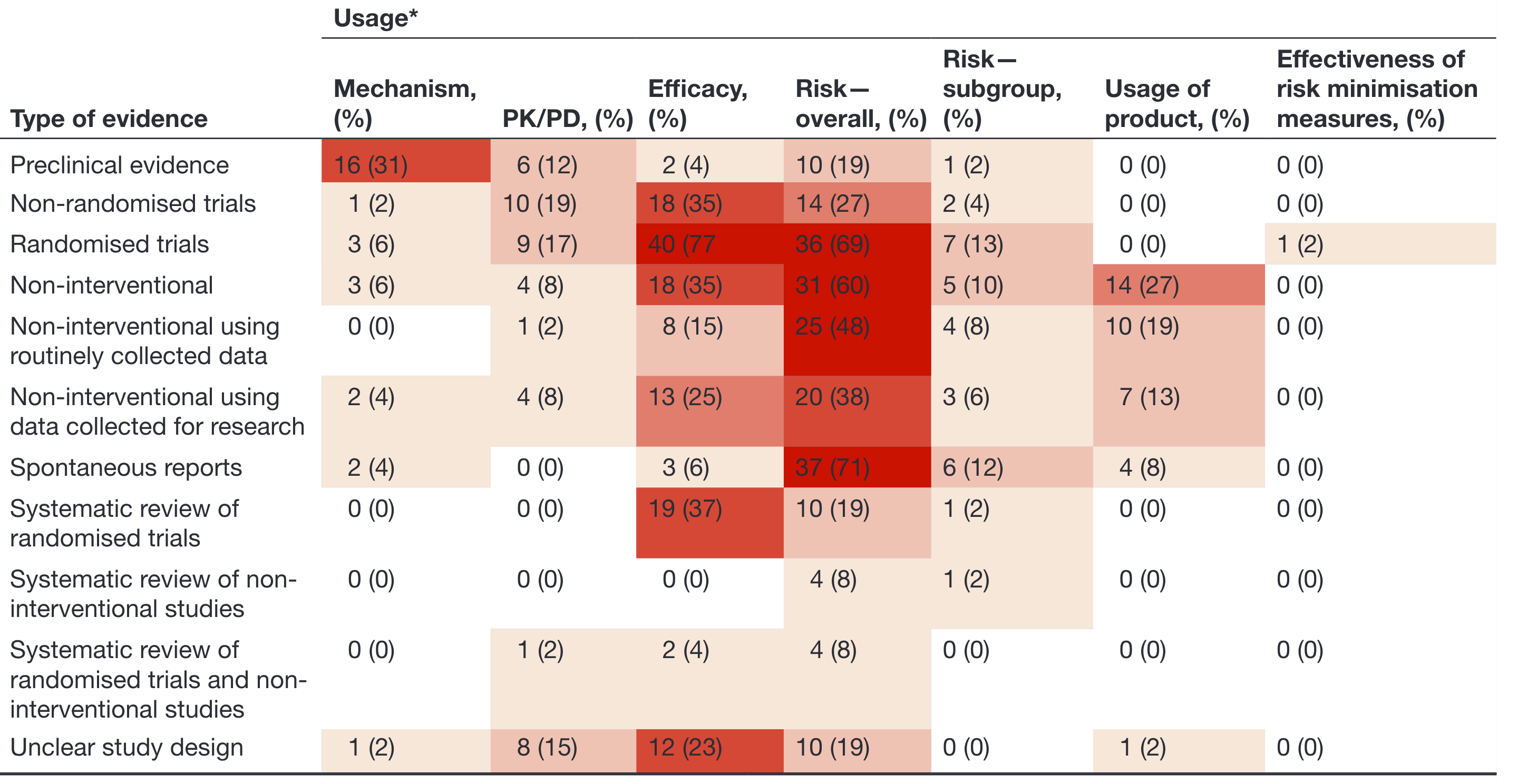
How is RWE used?
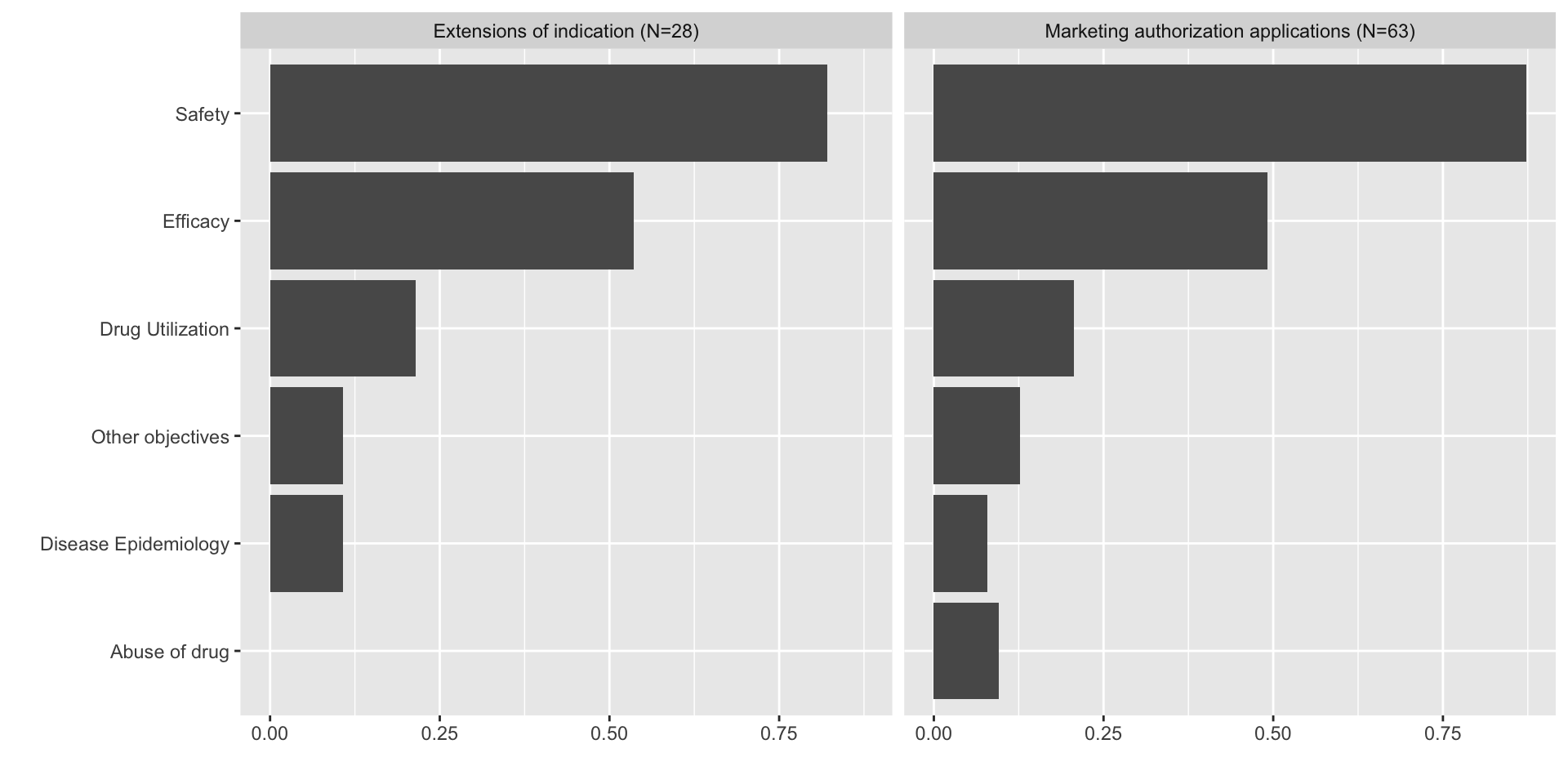
RWE studies - what is the catch?
- Selection bias
- Information bias
- Confounding
These biases may also appear in clinical trials, for instance when conducting subgroup analysis, principal stratum analysis, or indirect treatment comparisons.
Recommendations for RWE studies

Recommendations for RWE studies
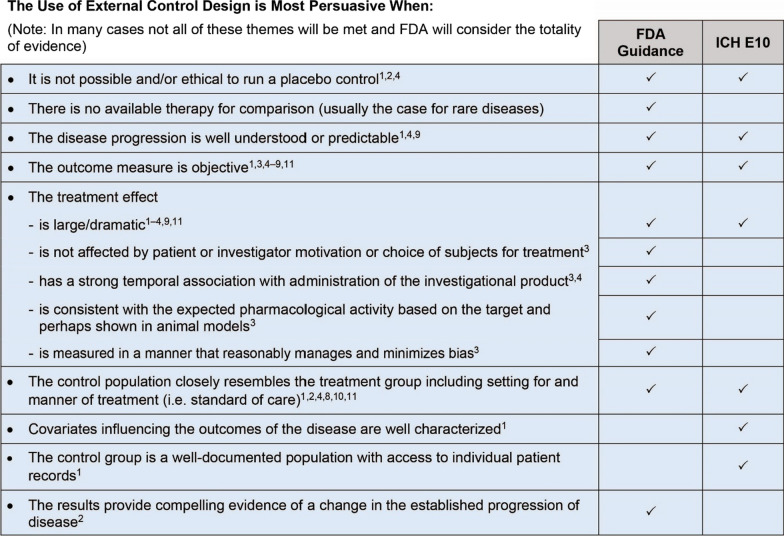
Recommendations for RWE studies
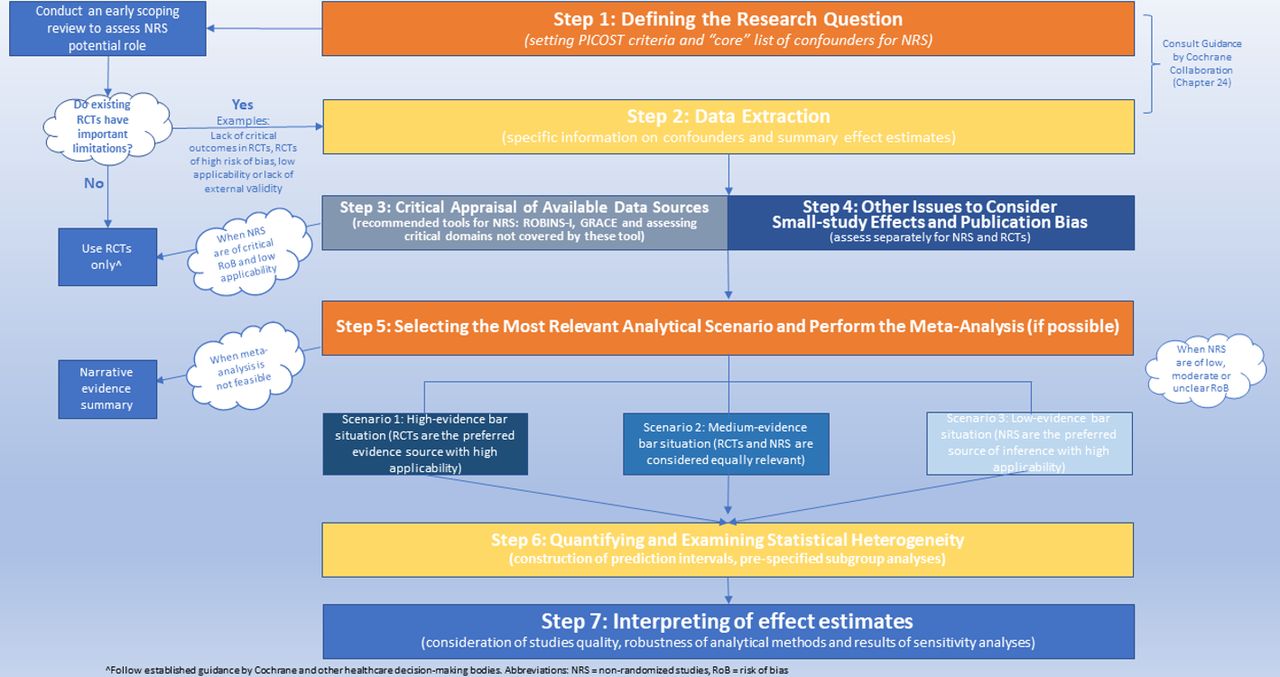
Recommendations for RWE studies
flowchart TD Z[Study planning] --> A(Defining the research question) A --> B(Planning study conduct) B --> C(Choosing fit-for-purpose data) C --> D(Primary data collection) C --> E[Study conduct] D --> E
flowchart TD Z[Study conduct] --> A(Choosing study design and analytical methods) A --> B(Minimising risk of bias) B --> C(Assessing robustness of study results) C --> D(Using proportionate quality assurance processes) D --> E[Study reporting]
Analysis of RWE studies
Statistical methods for addressing confounding
- Covariate adjustment
- Propensity score analysis
- Prognostic score analysis
These methods are also the building blocks for studies conducting indirect treatment comparisons or incorporating historical controls.
Analysis of RWE studies
Many other concerns should be explored:
- Operational definitions of key study variables
- Definition of time windows
- Choice of patient eligibility criteria
- Missing data and measurement error
- Alternative model specifications
- Treatment switching or loss to follow up
- Non-adherence
What is next?
Regulatory decision making

- The future is not about randomized controlled trials (RCTs) vs. real-world evidence (RWE) but about RCTs and RWE — not just for the assessment of safety but also of effectiveness
- Patients will need to be followed up for prolonged periods of time to empirically confirm or refute a priori comparative effectiveness assumptions
Requirements
- Need for additional sources of (non-RCT) evidence
- Need for better methods for evidence synthesis
- Need for revised regulatory (and other decision) frameworks
Data platforms

Catalogue of observational data sources for use in medicines regulation

Federated data network of allowing access to the data of EU citizens standardised to a common data model.
Individualizing treatment decisions
- Causal treatment effects are estimated at the population level in randomised controlled trials (RCTs)
- However, clinical decision is often to be made at the individual level in practice
- Individualized absolute treatment effects provide a natural starting point to engage in shared decision making
Estimating individualized treatment effects
- Subgroup analysis is often used to investigate heterogeneity in treatment effects
- Although subgroup effects can provide interesting insights and generate new hypotheses, they may be difficult to use for guiding treatment
- It may be more informative to move to the absolute risk scale and estimate multivariable models that adjust for individual patient characteristics
Estimating individualized treatment effects
- Small RCTs
- Hard to improve beyond risk-magnification
- However, the price to pay to allow for treatment-covariate interactions was small when using both shrinkage and selection, especially for the hierarchical group lasso
- Large RCTs
- Shrinkage and selection still needed
- Allowing for all interactions was beneficial
Estimating individualized treatment effects



precmed: Precision Medicine
A doubly robust precision medicine approach to fit, cross-validate and visualize prediction models for the conditional average treatment effect. Available from GitHub and CRAN.
Comparative effectiveness and personalized medicine using real-world data
- Edited by TPA Debray, T-L Nguyen and RW Platt
- 21 chapters; publication expected in 2024
- Contributions from ~ 40 authors (academia; industry; contract resesarch organizations; health technology assessment agencies; regulatory decision-making)
- Example code on GitHub


